Welcome to Akshar Shop
Liquid Ammonia
Liquid Ammonia
Cleaner. Household ammonia is a solution of NH3 in water (i.e., ammonium hydroxide) used as a general purpose cleaner for many surfaces. Because ammonia results in a relatively streak-free shine, one of its most common uses is to clean glass, porcelain and stainless steel.
We are one of the leading suppliers & wholesalers of Liquid Ammonia in Kolkata. We can offer you this product at a very competitive price in Kolkata.
What is Ammonia?
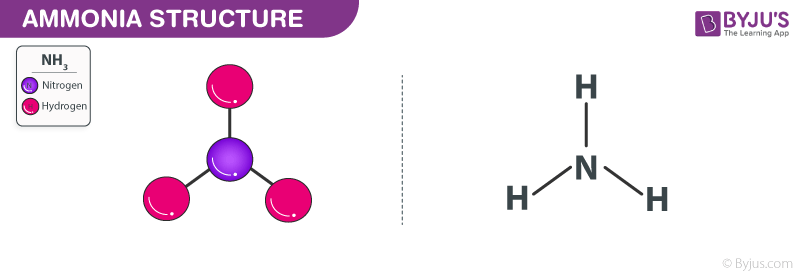
Ammonia is a colorless gas with a chemical formula NH3.
It consists of hydrogen and nitrogen. In its aqueous form, it is called ammonium hydroxide. This inorganic compound has a pungent smell. In its concentrated form, it is dangerous and caustic.
Ammonia is lighter than air with a density of 0.769 kg/m3 at STP. It is widely used as a fertilizer. It is also used in the manufacturing of explosives such as nitrocellulose and TNT. Also, it is used in the production of soda ash and in the Ostwald process to get nitric acid.
Ammonia Structure – NH3
Ammonia is known to behave as a weak base since it combines with many acids to form salts. For example, when it is reacted with hydrochloric acid, ammonia is converted into ammonium chloride. All the salts that are produced from such acid-base reactions are known to contain the ammonium cation, denoted by NH4+. It is interesting to note that ammonia also exhibits weak acidic qualities and can, therefore, be regarded as an amphoteric compound. The acidic qualities of ammonia enable it to form amides with some alkali metals and alkaline earth metals. An example of such a reaction can be observed when lithium is exposed to liquid ammonia, triggering the formation of lithium amide (a chemical compound with the formula LiNH2).
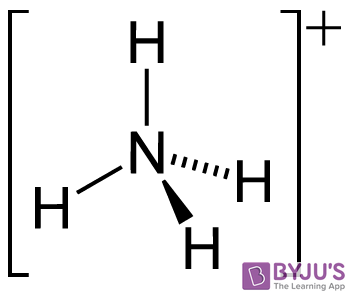
It can also be noted that the NH3 molecule undergoes self dissociation when dissolved in water. The molecular autoionization of the ammonia molecule results in the formation of its conjugate base (NH2–) and its conjugate acid (NH4+). The structure of the ammonium cation is illustrated below.
This autoionization process can be represented by the following equilibrium reaction:
2NH3 ⇌ NH2– + NH4+
Since ammonia generally functions as a relatively weak base, it can be used for buffering purposes (for the control of pH changes).
Preparation of Ammonia – NH3
Ammonia is easily made in the laboratory by heating an ammonium salt, such as ammonium chloride NH4Cl with a strong alkali, such as sodium hydroxide or calcium hydroxide.
2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3(g)
The gas may also is made by warming concentrated ammonium hydroxide.
The principal commercial method of production of ammonia is the Haber Process, the direct combination of nitrogen and hydrogen under high pressure in the presence of a catalyst.
Feature:
As a liquid, it is commonly diluted and used as a household cleaner. ... As a fertilizer, anhydrous ammonia gas is compressed into liquid and mixed with other plant growth enhancers. It can also be applied in gaseous form, where it combines with the moisture in the dirt, resulting in ammonia enriched fertilizer soil.
NH3 Uses (Ammonia)
- It is used as fertilizers as it increases the yield of crops
- It is used in the household as a cleaner – NH3 is mixed with water to clean stainless steel and glass
- It is used in food products as an antimicrobial agent
- It is used in the fermentation industry
- It is used as a refrigerant
- It is used as a pH adjuster in the fermentation process
- It is used to neutralize pollutant like nitrogen oxides emitted from diesel engines
- It is used as a fuel for rocket engines
- It is used in textile industries
- It is used in the manufacture of synthetic fiber like rayon and nylon
Additional
Logistics:
- Fast Delivery
- FOR Kolkata
- Delivery on Time
Packing Details:
Our Liquid Ammonia comes in following Packs:
- 250 ml
- 500 ml
- 1 lb
Payment Terms
Against order & Performa Invoice
Nature of Business: Suppliers, Manufacturers, Dealers, Exporters & Importers & Wholesalers | Area: Kolkata | Item Name: Liquid Ammonia